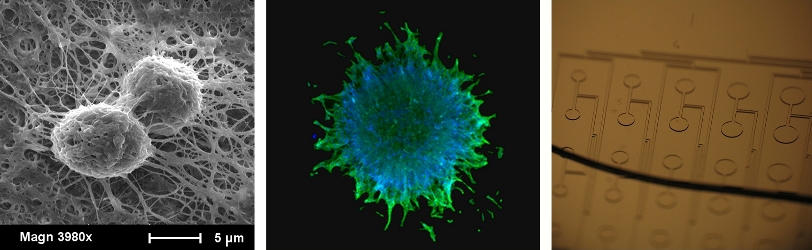
Posted on August 20, 2015 by chrismoraes
What started out as a disastrous experiment to make hand-pulled cotton candy in Chris’ kitchen turned into a pretty sweet technique to microfabricate structures in really soft silicone materials (published in Lab on a Chip last week). Generating structures in materials that have the consistency of watered-down pudding is particularly challenging, as peeling away a mold tends to destroy the structures. While experimenting with candy-making, instead of creating delectable, delicate and light sugar structures, Chris ended up with a hard lump of sugar that tasted horrible – but seemed to hold the microscale features of his Ziploc plastic container rather well. He raced that lump down to the lab, and after some modifications, found that (a) designer hard candies can hold microscale features, (b) that PDMS can be cast and cured against the candy, and (c) the candy could be gently removed by dissolving away in warm water. We then used microengineered soft molded structures to measure contraction of engineered microtissues, as a simple demonstration of this fabrication technology. Seems quite promising for many applications in soft matter engineering, and as an added bonus, the lab smells *delicious*.
[UPDATE: lots of news coverage on this piece! Check out the publications page for links]
Posted on June 2, 2015 by chrismoraes
Congratulations to Avital Horowitz, who won the best podium presentation award at the 2015 Quebec-Ontario Biotechnology meeting, for her talk on Candy-derived microstructures in soft silicone materials!
Posted on December 23, 2014 by chrismoraes
With apologies in advance for the massive number of puns we are about to inflict on the research world, Chris begins a new role as Highlights Editor for the RSC journal Integrative Biology! Check out the first of these bi-monthly reports of recent discoveries from labs that develop unique technological approaches to studying biological systems. This months’ highlight reports on integrating advanced imaging capabilities into microfluidic systems, to study biology without any pesky diffraction limitations in imaging resolution.
Posted on December 23, 2014 by chrismoraes
We know that 3D cultures can be more realistic than growing cells on hard, flat 2D surfaces. However, conducting high-throughput screens in 3D is extremely challenging from a technical, logistical and cost perspective. To address these issues, Brendan and Chris developed a micro-scale 3D culture assay that can be integrated directly into existing high-throughput screening infrastructure, is only slightly more expensive than conventional 2D assays, and eliminates the transport limitations typically associated with 3D systems. A preliminary screen of breast cancer cell chemo-responsiveness showed dramatic differences in chemotherapeutic activity between 2D and 3D cultures: check out the paper here and on our Publications page.
Posted on December 23, 2014 by chrismoraes
Gradients of soluble factors are known to drive key cellular processes such as migration. Establishing a soluble gradient can be done with microfluidics, but is a finicky process – accidental bumps can destroy a carefully-formed gradient. If this is challenging to do under controlled conditions, how do gradients form in the chaos of the human body? Using common microfabrication tools, Tai developed an ultra-simple 3D migration assay to probe this question in naturally-occuring hydrogel matrices. We then experimentally demonstrated that migration of cancer cells may be driven by gradients that self-assemble as a result of vastly increased binding interactions in ‘sticky’ 3D hydrogels. So not only do 3D environments influence cell function directly, they also shape the signals provided to the cells themselves, adding a new level of complexity to 3D studies of biological systems. For more information, see the full paper here, or on our Publications page.
Posted on May 12, 2014 by chrismoraes
Fabricating dynamic microfluidic structures can be challenging. In Byoung Choul and Chris’ latest work, adjustable microfluidic channels with defined dimensions and positions can be created by simply stretching and fracturing a multilayered material. In this technique, a film of brittle silicone is sandwiched between two tough silicone layers, and stretched. The modulus and toughness mismatch between the materials causes the brittle layer to fracture in a well-defined pattern. The fracture forms an adjustable microfluidic channel, which is completely reversible: it seems that breaking up is not that hard to undo. We used this technique to trap single cells within an adjustable microchannel, mechanically lyse them during channel collapse, and linearize the released nuclear chromatin for epigenetic analysis. Please see publications for more information.
Posted on April 21, 2014 by chrismoraes
Our work in printing contractile collagen droplets using aqueous-two phase technology was featured in Genetic Engineering News. Microengineered systems enable the study of biology in unique ways, but are challenging to implement without extensive training and experience. The technology described in our work (link) enables the rapid and simple production of collagen ‘microgels’ using equipment no more complex than a standard lab pipette. Microgels can be used to circumvent issues of limited availability of cell samples, and limited diffusion ranges of large molecules in collagen-based assays; the feature article highlights our use of these microgels to study contraction of collagen in response to external soluble signals.
Posted on March 8, 2014 by chrismoraes
In three-dimensional culture systems, cells are typically encapsulated in a complex, fibrous mesh-like structure. In Chris’ paper in Lab on a Chip (“Defined topologically-complex protein matrices to manipulate cell shape via three-dimensional fiber-like patterns”), we develop a technique to simplify and replicate three-dimensional fiber-like adhesive micropatterns for ‘minimalistic 3D cell culture’. This technology allows us to identify the impact of 3D matrix architectural features on cell function, which may then be used to inform biomaterial design for tissue engineering or regenerative medicine.
Posted on February 1, 2014 by chrismoraes
Our recent paper, “On being the right size: scaling effects in designing a human-on-a-chip” was selected as a research highlight by Lab on a Chip (link)! The paper presents and experimentally demonstrates some challenges, possibilities, and important considerations in the design of a multi-organ artificial cell culture system, or a ‘human-on-a-chip’, to screen new therapeutic compounds prior to expensive and time-consuming animal and human clinical trials.
Posted on January 27, 2014 by chrismoraes
<Throws the switch>
Cue suitable lightning effects.
Ominous roll of thunder.
It’s… alive!