Overview
Cells exist in complex microenvironments which provide distinct physical and chemical signals that drive cell function. These signals also guide cellular response to therapeutic agents. Hence, providing cells with physiologically realistic environments is a crucial precursor to testing and developing new therapies. Despite the known importance of realistic microenvironmental signals, the vast majority of cell culture studies are conducted on hard plastic Petri dishes; and while drug discovery strategies such as high-throughput screening have been immensely beneficial, this microenvironmental mismatch may explain the extraordinarily low success rates seen in translating seemingly promising therapies to actual clinical practice.
In order to bridge this gap and develop more realistic cell culture systems, we integrate fundamental expertise in chemical, mechanical and materials engineering with novel tissue engineering approaches to study biological systems in new and exciting ways. To date, we have focused on cardiovascular, cancer and pulmonary biology, with a specific focus on each of the following areas.
Mechanical forces in biological systems
Cells are now recognized as hubs of mechanical activity: responding to external mechanical signals, they generate forces, restructure the surrounding matrix, and coordinate the self-assembly of tissues and organs. Thinking of living tissues as being highly responsive, adaptable, and robust structures suggests unique opportunities to understand, manipulate, and learn from biological systems. To address some of the key challenges in understanding the role mechanical forces play in biological systems, we have developed novel microtechnologies capable of screening cellular response to cyclic mechanical loads in a high-throughput manner. We have also designed techniques to measure dynamic mechanical forces in three-dimensional developing tissues, and are currently applying these platform technologies to study disease progression in cardiovascular and cancer systems.
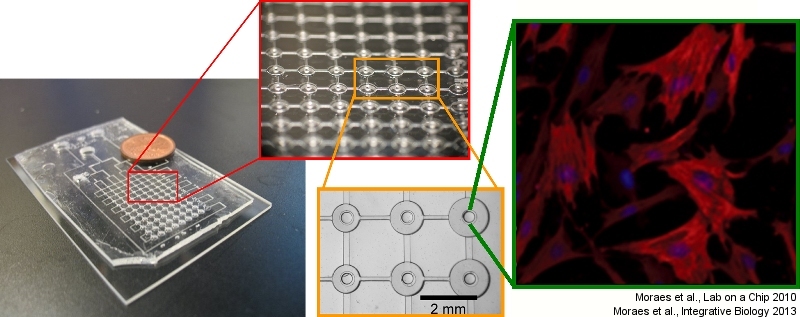
Microengineered biomaterials
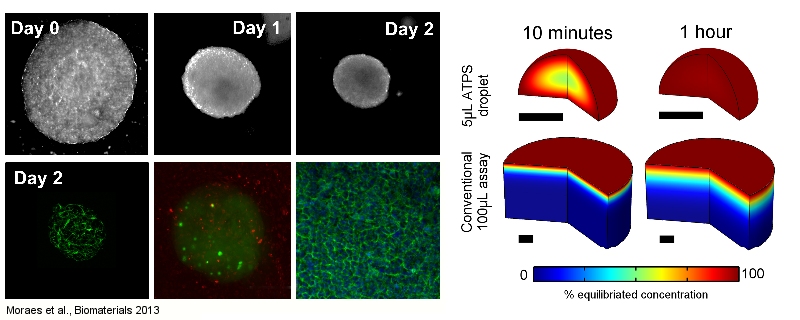
The rational design of biomaterials to direct cell function is a cornerstone of tissue engineering and regenerative medicine. Scientists are currently able to design biomaterials at the nano- (molecular) scale, and at the macro-scales to achieve these goals. We focus on designing technologies to control biomaterials at the interface of these two size regimes, and leverage the unique properties of such microengineered systems to address fundamental biological questions. In this area, we are developing novel and versatile techniques to study cells in three-dimensional environments, particularly in terms of controlling and manipulating the fibrous nature of 3D hydrogel environments at the single-cell level, and designing microscale tissues to circumvent transport limitations in conventional culture systems. We also design novel ‘smart’ biomaterial platforms that enable culture of cells in highly dynamic, but precisely-controlled microenvironments. These studies typically require the development of novel tissue engineering and biofabrication technologies in combination with fundamental engineering analyses, and can be of critical importance in tissue engineering applications.
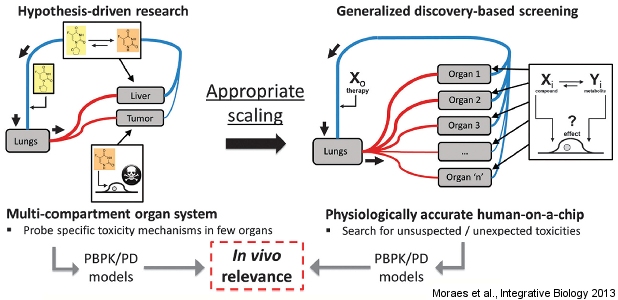
Next-generation drug discovery systems
Our ultimate goal is to create a complete miniaturized version of human physiology in a microfabricated format. This ‘human-on-a-chip’ concept has generated tremendous interest and excitement in the field, as it would enable highly realistic screening of new therapies, prior to expensive animal testing and lengthy human clinical trials. In working towards this, we are exploring the “design rules” necessary to develop complete biological discovery systems in which multiple cell types can be cultured in realistic proportions, in combination with integrated analytical techniques, to provide real-time and high-resolution feedback on physiological state.