Please note: Digital copies provided for personal use only.
Citation information available via Google Scholar.
Journal articles:
§, * indicates equal authorship
| [85]. | 3D Bioprinting of Food Grade Hydrogel infused with Living Pleurotus ostreatus mycelium in Non-Sterile Conditions Lin, N., Taghizadehmakoei, A., Polovina, L, McLean, I., Santana-Martinez, J.C., Naese, C., Moraes, C., Hallam S.J.*, Dahmen, J.* ACS Applied Bio Materials, in press (2024) [pdf] [link] |
|
| [84]. | Engineering placental trophoblast fusion: a potential role for biomechanics in syncytialization Parameshwar, P.K., Vaillancourt, C., Moraes, C.*. Placenta, special issue on Trophoblast Research (DOI: 10.1016/j.placenta.2024.02.006) [pdf] [link] |
|
| [83]. | Computational and experimental investigation of the effect of surface topography and cellular biomechanics on the nanopillar-induced bactericidal mechanism Valiei, A.*, Bryche, J.F., Canva, M. Charette, P.G., Moraes, C., Hill, R.J.*, Tufenkji, N., ACS Applied Materials & Interfaces, 16, 8, 9614–9625 (2024) |
|
| [82]. | Development of Photocrosslinkable Bioinks with Improved Electromechanical Properties for 3D Bioprinting of Cardiac BioRings Mousavi A., Hedayatnia, A., Van Vliet P., Dartora, D.R., Wong N., Rafatian, N., Nuyt, A.M., Moraes C., Ajji A., Andelfinger G., Savoji H*., Applied Materials Today 36 (2024) [pdf] [link] |
|
| [81]. | Formulation and evaluation of PVA/Gelatin/Carrageenan inks for 3D printing and development of tissue-engineered heart valves Jafari, A., Niknezhad, S.V., Kaviani, M., Saleh, W., Piet Van Vliet, P., Wong, N., Moraes, C., Ajji, A., Kadem, L., Azarpira, N., Andelfinger, G., Savoji, H., Advanced Functional Materials (DOI: 10.1002/adfm.202305188) [pdf] [link] |
|
| [80]. | Monitoring Bioluminescent Pseudomonas aeruginosa on Mechano-Bactericidal Zinc Oxide Nanopillars: Implications for Self-Cleaning Antibacterial Coatings Lin, N., McKay, G., Dao, N., Moraes, C.*, Tufenkji, N.*, ACS Applied Nano Materials, DOI: 10.1021/acsanm.3c03789 (2023) [pdf] [link] |
|
| [79]. | pH-responsive reversible granular hydrogel based on metal-binding mussel-inspired peptides Rammal, M., Li, C., Reeves, J., Moraes, C., Harrington, M., ACS Applied Materials and Interfaces DOI: 10.1021/acsami.3c06013 (2023) [pdf] [link] |
|
| [78]. | Electrochemical removal of bacteria from zinc oxide nanopillars synthesized on stainless steel Lin, N., Nguyen, B., Omanovic, S., Moraes, C.*, Tufenkji, N.*, ACS Applied Engineering Materials 1,1524−1534 (2023). [pdf] [link] |
|
| [77]. | Substrate viscoelasticity affects human macrophage morphology and phagocytosis Kalashnikov, N., Moraes, C., Soft Matter DOI: 10.1039/D2SM01683D (2023) [pdf] [link] |
|
| [76]. | Compressive molding of engineered tissues via smart material hydrogel devices Cassel de Camps, C., Mok, S., Ashby, E., Li, C., Lepine, P., Durcan, T.M., Moraes, C., Lab on a Chip DOI: 10.1039/D3LC00007A (2023) [pdf] [link] |
|
| [75]. | Ultrasoft edge-labelled hydrogel sensors reveal internal tissue stress patterns in invasive engineered tumors Lee, W., Boghdady, C-M., Lelarge, V., Leask, R.L., McCaffrey, L., Moraes, C.*, Biomaterials DOI: 10.1016/j.biomaterials.2023.122073 (2023) [pdf] [link] |
|
| [74]. | Integrating mechanical sensor readouts into organ-on-a-chip platforms Morales, I.A., Boghdady, C-M., Campbell, B.E., Moraes, C., Frontiers in Bioengineering and Biotechnology DOI: 10.3389/fbioe.2022.1060895 (2023) [pdf] [link] |
|
| [73]. | Decellularized extracellular matrix: New promising and challenging biomaterials for regenerative medicine Brown, M., Li, J., Moraes, C., Tabrizian M., Li-Jessen, N.Y., Biomaterials DOI: 10.1016/j.biomaterials.2022.121786 (2022) [pdf] [link] |
|
| [72]. | Engineering physical microenvironments to study innate immune cell biophysics Kalashnikov, N., Moraes, C., APL Bioengineering, DOI: 10.1063/5.0098578 [pdf] [link] |
|
| [71]. | Engineered models for placental toxicology: emerging approaches based on tissue decellularization Parameshwar, P.K.*, Sagrillo-Fagundes, L.*, Portilho, N.A., Pastor, W.A., Vaillancourt, C., Moraes, C., Reproductive Toxicology DOI: 10.1016/j.reprotox.2022.07.003 (2022) [pdf] [link] |
|
| [70]. | Microfluidic study of bacterial attachment on and detachment from zinc oxide nanopillars Lin, N., Valiei, A., McKay, G., Nguyen, D., Tufenkji, N.*, Moraes, C.*, ACS Biomaterials Science & Engineering DOI: 10.1021/acsbiomaterials.2c00233 [pdf] [SI] [link] |
|
| [69]. | Surface wettability is a key feature in the mechano-bactericidal activity of nanopillars Valiei, A., Lin, N., McKay, G., Nguyen, D., Moraes, C., Hill, R.J., Tufenkji, N ACS Applied Materials & Interfaces DOI: 20.1021/acsami.2c03258 [pdf] [SI] [link] |
|
 |
[68]. | The case for cancer-associated fibroblasts: essential elements in cancer drug discovery? Brewer, G., Fortier, A-M., Park, M., Moraes, C., Future Drug Discovery, doi:10.4155/fdd-2021-0004 [pdf] [link] |
 |
[67]. | Hydrogel mechanics influence growth and development of embedded brain organoids Cassel de Camps, C., Aslani, S., Stylianesis, N., Nami, H., Mohamed, N-V., Durcan, T.M*, Moraes, C.* ACS Applied Bio Materials (in press) [pdf] [SI] [link] |
 |
[66]. | Accessible, large-area, uniform dose photolithography using a moving light source Kaltashov, A.*, Parameshwar, P.K.*, Lin, N.*, Moraes, C., Journal of Micromechanics and Microengineering [pdf] [SI] [link] |
| [65]. | The DNMT1 inhibitor GSK-3484862 mediates global demethylation in murine embryonic stem cells Portilho, N.A., Saini, D., Hossain, I., Sirois, J., Moraes, C., Pastor W.A., Epigenetics and Chromatin (in press) [pdf] [SI] [link] |
|
 |
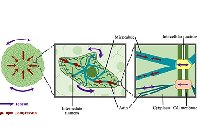
[64]. | Disease-specific extracellular matrix composition regulates placental trophoblast fusion efficiency Parameshwar, P.K., Sagrillo-Fagundes, L., Fournier, C., Girard, S., Vaillancourt C., Moraes, C., Biomaterials Science, DOI: 10.1039/D1BM00799H (2021) [pdf] [SI] [link] |
 |
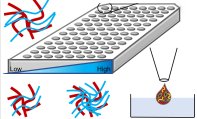
[63]. | Revisiting tissue tensegrity: biomaterial-based approaches to measure forces across the length scales Boghdady, C-M., Kalashnikov, N., Mok, S., McCaffrey, L., Moraes, C., APL Bioengineering 5, 041501 (2021) [pdf] [link] *Selected as a featured article by the journal editors |
 |
[62]. | Bioprintable, stiffness-tunable collagen-alginate microgels for increased throughput 3D cell culture studies Ort, C.A., Chen, Y., Ghagre, A., Ehrlicher, A.J., Moraes, C., ACS Biomaterials Science & Engineering, DOI: 10.1021/acsbiomaterials.1c00129 (2021) [pdf] [SI] [link] |
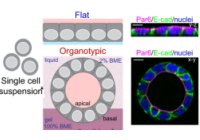 |
[61]. | Architectural control of metabolic plasticity in epithelial cancer cells Al-Masri, M., Paliotti, K., Tran, R., Halaoui, R., Lelarge, V., Chatterjee, S., Wang, L-T., Moraes, C., McCaffrey, L. Communications Biology 4, 371 (2021) [link] |
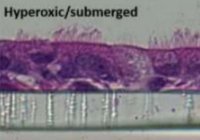 |
[60]. | Oxygenation as a driving factor in epithelial differentiation at the air-liquid interface Kouthouridis, S., Goepp, J., Martini, C., Matthes, E., Hanrahan, J.W., Moraes, C., Integrative Biology 13, (3) p61-72 (2021) [pdf] [link] |
 |
[59]. | Accessible dynamic micropatterns in monolayer cultures via modified desktop xurography Tran, R., Hoesli, C.A*., Moraes, C.* Biofabrication 13 025003 (2021) [pdf] [SI] [link] |
 |
[58]. | Disentangling the fibrous microenvironment: designer culture models for improved drug discovery Ort, C.A., Lee, W., Kalashnikov N., Moraes, C. Expert Opinion on Drug Discovery 20 (8), DOI: 10.1080/17460441.2020.1822815 (2021) [pdf] [link] |
 |
[57]. | Developmentally-inspired biomimetic culture models to produce functional islet-like cells from pluripotent precursors Tran., R., Moraes, C*, Hoesli, C.A.* Frontiers in Bioengineering and Biotechnology 8:583970 (2020) [pdf] [link] |
 |
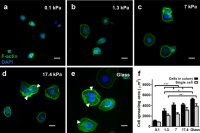
[56]. | Mapping cellular-scale internal mechanics in 3D tissues with thermally responsive hydrogel probes Mok, S., Al Habyan, S., Ledoux, C., Lee, W., Macdonald, K.N., McCaffrey, L., Moraes, C. Nature Communications 11, 4757 (2020) [link] [SI] [in the media: McGill NewsRoom; en Francais; McGill Tribune; altmetric] |
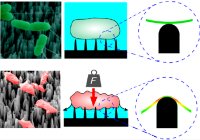 |
[55]. | Hydrophilic Mechano-Bactericidal nanopillars require external forces to rapidly kill bacteria Valiei, A., Lin, N., Bryche, J.F., McKay, G., Canv, M., Charette, P.G., Nguyen, D., Moraes, C*, Tufenkji, N* Nano Letters 20 (8), 5720–5727 (2020) [pdf] [SI] [link] |
 |
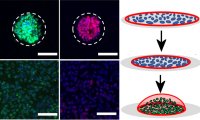
[54]. | Mechanobiological regulation of placental trophoblast fusion and function through extracellular matrix rigidity Ma, Z., Sagrillo-Fagundes, L., Mok, S., Vaillancourt, C., Moraes, C., Scientific Reports 10, 5837 (2020) [pdf] [link] |
 |
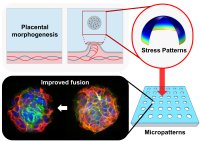
[53]. | Enhanced differentiation of pluripotent stem cell-derived pancreatic progenitors using confined micropatterned cultures Tran, R., Moraes, C.*, Hoesli, C.A.*, Scientific Reports 10:1190 (2020) [pdf] [SI] [link] |
 |
[52]. | Biomimetic micropatterned adhesive surfaces to mechanobiologically regulate placental trophoblast fusion Ma, Z., Fagundes, L.S., Tran, R., Parameshwar, P.K., Kalashnikov, N., Vaillancourt, C., Moraes, C., ACS Applied Materials & Interfaces 2019 [pdf] [link] |
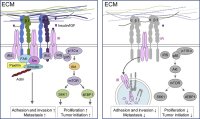 |
[51]. | Functional redundancy between Beta1 and Beta3 Integrin in Activating the IR/Akt/mTORC1 signaling axis to promote ErbB2-Driven Breast Cancer Bui, T., Rennhack, J., Mok, S., Ling, C., Perez, M., Roccamo J., Andrecheck, ER, Moraes, C., Muller W.J. Cell Reports 29 (3) 589-602 2019 [link] |
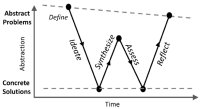 |
[50]. | The W-Model: A pre-college design pedagogy for solving wicked problems Moraes, C.§, Blain-Moraes, S.§, Morell-Tomassoni, S., Gorbet, R. International Journal of Technology and Design Education 2019 [pdf] [link] |
 |
[49]. | Robust and precise wounding and analysis of engineered contractile tissues. Dubois, S.J. §, Kalashnikov, N. §, Moraes, C. Tissue Engineering C 2019 [pdf] [SI] [link] |
 |
[48]. | Magnetic microboats for floating, stiffness tunable, air liquid interface epithelial cultures Chandrasekaran, A., Kouthouridis, S., Lee, W., Lin, N., Ma, Z., Turner, J.M., Hanrahan, J.W., Moraes, C., Lab on a Chip 2019 DOI:10.1039/c9lc00267g [pdf] [SI] [link] |
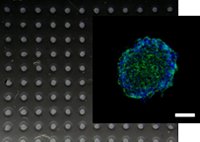 |
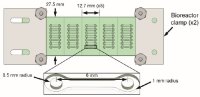
[47]. | Micropocket hydrogel devices for all-in-one formation, assembly, and analysis of aggregate-based tissues Zhao, L., Mok, S., Moraes, C., Biofabrication 2019 DOI: 10.1088/1758-5090/ab30b4 [pdf] [SI] [link] |
 |
[46]. | Dynamic bioreactors with integrated microfabricated devices for mechanobiological screening Beca, B.M., Sun, Y., Wong, E., Moraes, C*., Simmons, C.A.* Tissue Engineering C 2019 [pdf] [link] |
 |
[45]. | Morphodynamic tissues via integrated programmable shape memory actuators Kalashnikov, N., Moraes, C., Advanced Functional Materials 2019, 1903327 [pdf] [SI] [link] |
 |
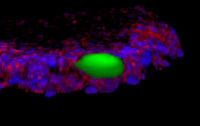
[44]. | Microfluidic Shear Assay to Distinguish between Bacterial Adhesion and Attachment Strength on Stiffness-Tunable Silicone Substrates Siddiqui, S., Chandrasekaran, A., Lin, N., Tufenkji, N., Moraes, C., Langmuir 2019, DOI:10.1021/acs.langmuir.9b00803. [pdf] [SI] [link] |
 |
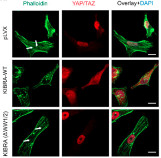
[43]. | Dispersible hydrogel force sensors reveal patterns of solid mechanical stress in multicellular spheroid cultures Lee, W., Kalashnikov, N., Mok, S., Halaoui, R., Kuzmin, E., Putnam, A.J., Takayama, S., Park, M., McCaffrey L., Zhao, R., Leask, R.L., Moraes, C., Nature Communications 2019, 10:144. [pdf] [SI] [link] [In the press: McGill NewsRoom] |
 |
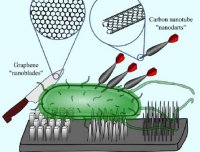
[42]. | Kibra (WWC1) is a metastasis suppressor gene affected by chromosome 5q loss in human triple negative breast cancers. Knight JF, Sung V, Kuzmin E, Couzens A, de Verteuil DA, Johnson RM, Gruosso T, Lee W, Saleh SM, Zuo D, Guiot MC, Davis RR, Zhao H, Gregg JP, Moraes C, Gingras AC, Park M., Cell Reports 2018, 22 (12) p. 3191-32015 [link] |
 |
[41]. | Nanodarts, nanoblades, and nanospikes: Mechano-bactericidal nanostructures and where to find them Lin, N., Berton, P., Moraes, C., Rogers, R.D., Tufenkji, N., Advances in Colloid and Interface Science, 2018, 252, p. 55-68 [pdf] |
 |
[40]. | Dispersible oxygen microsensors map oxygen gradients in three-dimensional cell cultures Lesher-Perez, S.C., Kuo, C-H., Leung, B.M., Kim, G-H., Mong, S., Kojima, T., Moraes, C., Thouless, M.D., Luker, G.D., Takayama, S., Biomaterials Science, 2017 [pdf] |
 |
[39]. | Thermal scribing to prototype plastic microfluidic devices, applied to study the formation of neutrophil extracellular traps Chandrasekaran, A., Kalashnikov, N., Rayes, R., Wang, C., Spicer, J., Moraes, C., Lab on a Chip 2017, 17, 2003 – 2012 [pdf] [SI] [link] |
 |
[38]. | Building an experimental model of the human body with non-physiological parameters Labuz J.M., Moraes, C., Mertz, D., Leung, B.M., Takayama, S. Technology 2017, 05 (1), 42-59 [pdf] [link] |
| [37]. | Gotta catch ’em all: the microscale quest to understand cancer biology Ma., Z., Moraes, C., Integrative Biology, 2016, DOI: 10.1039/C6IB90045C [Research Highlight – link] |
|
| [36]. | Microfluidics in Microbiology: Putting a Magnifying Glass on Microbes Siddiqui, S.K., Tufenkji, N., Moraes, C., Integrative Biology, 2016, 8, 914-917 [Research Highlight – link] |
|
| [35]. | Stem Cells: to be born great, achieve greatness, or have greatness thrust upon them? Tran, R., Hoesli, C.A., Moraes, C., Integrative Biology, DOI: 10.1039/c6ib90021f [Research Highlight – link] |
|
 |
[34]. | Have microfluidics delivered for drug discovery? Chandrasekaran, A., Abduljawad, M., Moraes, C., Expert Opinion on Drug Discovery 2016 DOI: 10.1080/17460441.2016.1193485 [pdf] |
| [33]. | Next generation tools to accelerate the synthetic biology process Shih, S.C.C., Moraes, C. Integrative Biology2016 DOI: 10.1039/c6ib90017h [Research Highlight – link] |
|
| [32]. | Thinking big by thinking small: advances in mechanobiology across the length scales. Mok, S., Moraes, C., Integrative Biology 2016, 8, 262-266. [Research Highlight – link] |
|
| [31]. | Getting there is half the battle: recent advances in delivering therapeutics. Lesher-Perez, S.C., Segura, T., Moraes, C., Integrative Biology 2016. DOI: 10.1039/C5IB90052B [Research Highlight – link] |
|
| [30]. | Making it stick: the role of structural design in implantable technologies. Lee, W., Leask, R.L., Moraes, C., Integrative Biology2015. DOI: 10.1039/C5IB90041G [Research Highlight – link] |
|
| [29]. | Patients are a virtue: advances in microengineered systems for clinical applications. Young, E.W.K., Moraes, C. Integrative Biology 2015. DOI: 10.1039/C5IB90031J [Research Highlight – link] |
|
 |
[28]. | Supersoft lithography: Candy-based fabrication of soft silicone microstructures. Moraes, C., Labuz, J.M., Shao, Y., Fu, J., Takayama, S. Lab on a Chip 2015. DOI: 10.1039/C5LC00722D [pdf] [SI] [link] [News: The Sweet Smell of Science, U. Michigan Engineering Youtube video; Michigan Daily; PopSci.com; Smithsonian Magazine] |
| [27]. | Between a rock and a soft place: recent progress in understanding matrix mechanics. Moraes, C., Integrative Biology 2015 [Research Highlight – link] | |
| [26]. | Live long and prosper: the enterprise of understanding diseased epithelium. Horowitz, A., Moraes, C. Integrative Biology 2015 7, 494-497 [Research Highlight – link] |
|
| [25]. | The Discovery Channel: microfluidics and microengineered systems in drug screening. Moraes, C., Integrative Biology 2015 7, 285-288 [Research Highlight – link] |
|
| [24]. | Micro, Soft, Windows: Integrating super-resolution viewing capabilities into soft lithographic devices. Moraes, C., Integrative Biology 2015, 7 (1) 10-13 [Research Highlight – link] |
|
 |
[23]. | Microscale 3D collagen cell culture assays in conventional flat-bottom 384-well plates. Leung, B.M.§, Moraes, C.§, Cavnar, S.P., Luker, K.E., Luker, G.D., Takayama, S. Journal of Laboratory Automation DOI: 10.1177/2211068214563793 [pdf][SI] |
 |
[22]. | Surface-templated hydrogel patterns prompt matrix-dependent migration of breast cancer cells towards chemokine-secreting cells. Kojima, T., Moraes, C., Cavnar, S.P., Luker, G.D., Takayama, S. Acta Biomaterialia DOI: 10.1016/j.actbio.2014.11.033 [pdf][SI] |
 |
[21]. | Media additives to promote spheroid circularity and compactness in hanging drop platforms Leung, B.M., Lesher-Perez, S.C., Matsuoka, T., Moraes, C., Takayama, S. Biomaterials Science DOI: 10.1039/C4BM00319E [pdf] |
 |
[20]. | Fracture-based fabrication of normally-closed, adjustable and fully reversible micro-scale fluidic channels. Kim, B.C.§, Moraes, C.§, Huang, J., Matsuoka, T., Thouless, M.D., Takayama, S. Small 10 (19) pp. 4020-4029. [pdf][SI] |
 |
[19]. | Defined topologically-complex protein matrices to manipulate cell shape via three-dimensional fiber-like patterns. Moraes, C., Kim, B.C., Zhu, X., Mills, K.L., Dixon, A.R., Thouless, M.D., Takayama, S. Lab on a Chip 14, pp. 2191-2201 (2014). [pdf][SI] |
 |
[18]. | Biological applications enabled by fracture-guided micro and nanofabrication Kim, B.C., Moraes, C., Huang, J., Thouless, M.D., Takayama, S. Biomaterials Science 2, 288 (2014) [pdf] |
 |
[17]. | Guided fracture of films on soft substrates to create micro/nano-feature arrays with controlled periodicity Kim, B.C., Matsuoka, T., Moraes, C., Huang, J., Thouless, M.D., Takayama, S. Scientific Reports 3, 3027 (2013). [pdf] |
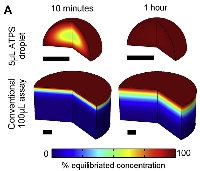 |
[16]. | Aqueous two-phase printing of contractile collagen microgels Moraes, C., Simon, A.B., Putnam, A.J., Takayama, S. Biomaterials 34 (37), pp. 9623-31 (2013). [pdf] [SI] [Highlight: Genetic Engineering and Biotechnology] |
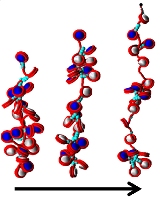 |
[15]. | Micro- and nanofluidic technologies for epigenetic profiling Matsuoka, T., Kim, B.C., Moraes, C., Hun, M., Takayama, S. Biomicrofluidics 7, 041301 (2013). [pdf] |
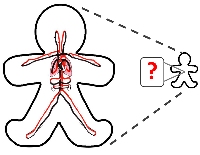 |
[14]. | On being the right size: scaling effects in designing a human-on-a-chip Moraes, C. §, Labuz M.J. §, Leung, B.M. §, Inoue, M., Chun, T-H., Takayama, S. Integrative Biology 5 (9), pp. 1149-61. (2013). [pdf] [Highlight: Lab on a Chip] |
 |
[13]. | One-dimensional patterning of cells in silicone wells via compression-induced fracture Dixon, A.R., Moraes, C., Csete, M., Thouless, M.D., Takayama, S. Journal of Biomedical Materials Research A 102(5), pp. 1361-9 (2013). [pdf] |
 |
[12]. | Microdevice array-based identification of distinct mechanobiological response profiles in layer-specific valve interstitial cells Moraes, C. §, Likhitpanichkul, M. §, Lam, C.J., Beca, B.M., Sun, Y., Simmons, C.A. Integrative Biology 5 pp. 673-80. (2013) [pdf] [SI] |
 |
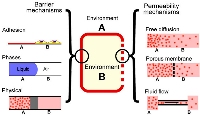
[11]. | Pop culture: a soap-bubble based framework for nanoeducation outreach Moraes, C. International Journal of Engineering Education 28(5) pp. 1088-94 (2012) [pdf] |
 |
[10]. | Organs on a chip: focus on compartmentalized microdevices Moraes, C. §, Mehta, G. §, Lesher-Perez, S. §, Takayama, S. Annals of Biomedical Engineering 40(6) pp. 1211-27 (2011) [pdf] |
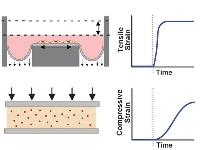 |
[9]. | (Micro) managing the mechanical microenvironment Moraes, C., Sun, Y., Simmons, C.A. Integrative Biology 3, pp. 959-971 (2011). [pdf] [Featured: Integrative Biology’s Top 2013 Cited Papers] |
| [8]. | Semi-confined compression of microfabricated biomaterial constructs Moraes, C., Zhao, R., Likhitpanichkul, M., Simmons, C.A., Sun, Y. Journal of Micromechanics and Microengineering (21) 054014 (2011). [pdf] |
|
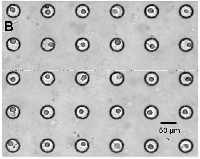 |
[7]. | Single cell deposition and patterning with a robotic system Lu, Z.§, Moraes, C.§, Ye, G., Simmons, C.A., Sun, Y. PLoS ONE, 5, e13542 (2010). [pdf][correction] |
 |
[6]. | Microfabricated platforms for mechanically dynamic cell culture Moraes, C., Sun, Y., Simmons, C.A. Journal of Visualized Experiments 46 (2) e2224 doi:10.3791/2224 (2010). [link] |
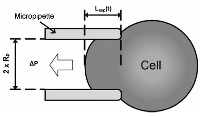 |
[5]. | An undergraduate lab (on-a-chip): Probing single cell mechanics on a microfluidic platform Moraes, C., Wyss, K., Brisson, E., Keith, B.A., Sun, Y., Simmons, C.A. Cellular and Molecular Bioengineering 3(3), pp. 319-330 (2010). [pdf] [labManual] |
 |
[4]. | Microfabricated arrays for high-throughput screening of cellular response to cyclic substrate deformation Moraes, C., Chen, J-H., Sun, Y., Simmons, C.A. Lab on a Chip 10(2) pp. 227-34 (2010). [pdf] |
 |
[3]. | A microfabricated platform for high throughput unconfined compression of micropatterned biomaterial arrays Moraes, C., Wang, G., Sun, Y., Simmons, C.A. Biomaterials 31(3) pp. 557-84 (2010). [pdf] |
 |
[2]. | Integrating polyurethane culture substrates into poly(dimethylsiloxane) microdevices Moraes, C., Kagoma, Y.K., Beca, B.M., Tonelli-Zasarsky, R.L.M., Sun, Y., Simmons, C.A. Biomaterials 30(28) pp. 5241-50 (2009). [pdf] |
 |
[1]. | Solving the shrinkage-induced PDMS registration problem in multilayer soft lithography Moraes, C., Sun, Y., Simmons, C.A. Journal of Micromechanics and Microengineering (19) 065015 (2009). [pdf] |
Book Chapters
[8]. Tran, R., Moraes, C., Hoesli, C.A., “Production of pluripotent stem cell-derived pancreatic cells by manipulating cell-surface interactions”, Advanced Materials, 2019, ISBN: 978-3-11-053765-9, in press.
[7]. Cooper, S., Moraes, C. §, Leask, R.L.,§ “Biological Considerations: Cardiovascular 3D systems” in “Engineering 3D Tissue Test Systems”, Taylor and Francis.
[6]. Leung, B.M., Labuz, J.M., Moraes, C., Takayama, S., 2015. “Chapter 9: Bioprinting using aqueous two phase systems” in Essentials of 3D Biofabrication and Translation, Elsevier ISBN: 978-0128009727
[4]. MacQueen, L., Moraes, C., Sun, Y., Simmons, C.A., 2014. “Chapter 16: Dynamic Mechanical Environments to Quantify and Control Cellular Dynamics” in Cells, Forces and the Microenvironment Pan Stanford Publishing, ISBN (Hardcover): 978-981-4613-36-1 ISBN (eBook): 978-981-4613-37-8).
[3]. Liu, J. §, Moraes, C. §, Lu, Z. §, Simmons, C.A., Sun, Y., 2012. “Chapter 23: Single cell deposition” in Methods in Cell Biology, 2012; 112:403-420, Elsevier.
[2]. White, J., Douville, N., Moraes, C., Takayama, S., 2011. “Microfluidic approaches towards pulmonary tissue constructs”, in Microfluidic Cell Culture Systems, Chapter 10, Elsevier 2011 (ISBN: 978-1-4377-3459-1)
[1]. Moraes, C., Sun, Y., Simmons, C.A., 2011. “Micro and nano-technologies for studies in cellular mechanics and mechanobiology”, in “Cellular and Biomolecular Mechanics and Mechanobiology”. Editor: Amit Gefen, Ph.D.; Book series: Studies in mechanobiology Tissue Engineering and Biomaterials, Vol 4, pp 145-175, DOI 10.1007/8415_2010_24
Other Contributions
[2]. Moraes, C., Sun, Y., Simmons, C.A., 2010. “Connector-less manipulation of small liquid volumes in microchannels” in Chips & Tips, (online supplement to Lab on a Chip). [link]
[1]. Moraes, C., Simmons, C.A., Sun, Y., 2006. “Cell Mechanics Meets MEMS”, Canadian Society of Mechanical Engineers Fall 2006 Bulletin. [pdf]